Data Integration for Medical Equipment Manufacturing
Connect quality management, manufacturing, and regulatory systems with validated data pipelines. Maintain FDA compliance while accelerating analytics and reporting.
Industry-Specific Outcomes
QMS to analytics for quality trend analysis
Manufacturing data warehouses for production insights
Automated regulatory submission pipeline workflows
Supply chain traceability for UDI compliance
Post-market surveillance integrated with quality systems
Popular Connector Workflows
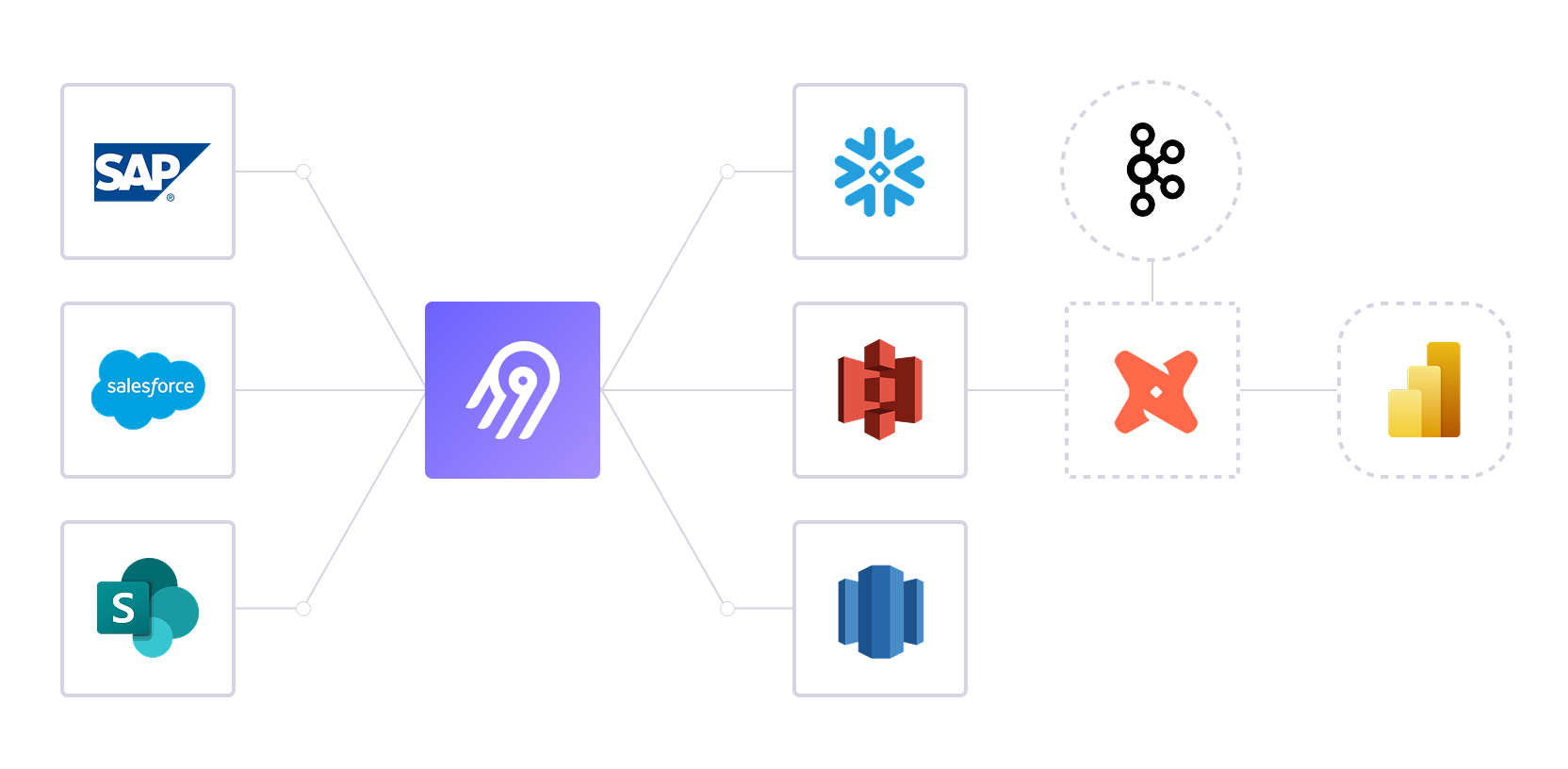
Medical Device Data Pipeline Architecture
Validated data flows from QMS, MES, and ERP systems through Airbyte to analytics platforms with complete audit trails.
Without such an integrated 360-degree view of customer engagement data, it was challenging for internal product teams to reach the right customers at the right time through push notifications or email messages. With Airbyte, we were able to save up to 10% of the marketing budget. In addition, the savings obtained with Airbyte helps the company reinvest into the business to lead to a higher return on marketing investment.

Compliance Considerations
FDA 21 CFR Part 11 with audit trails
ISO 13485 quality management compliance
HIPAA security for PHI protection
EU MDR/IVDR regulatory requirements
Recommended Connectors
See all connectorsSalesforce
CRM for managing hospital and healthcare provider relationships
Netsuite
ERP for manufacturing operations, inventory, and supply chain management
QuickBooks
Financial management and accounting data
Zendesk Support
Customer support tickets from healthcare clients and distributors
Snowflake destination
Data warehouse for analytics and regulatory compliance reporting
BigQuery
Cloud data warehouse for business intelligence and manufacturing insights
Related Resources
HIPAA Cloud Data Integration Compliance
Healthcare Data Integration FHIR Airbyte AI Assistant
Data Quality Issues
Supply Chain Data Management: A Complete Guide

Accelerate Compliant Data Integration
Move faster with secure, compliant, and open-source data integration.
